Beyond Compartment models: Using Big-Data to enhance models for controller development for the Artificial Pancreas
Project website
Project Description
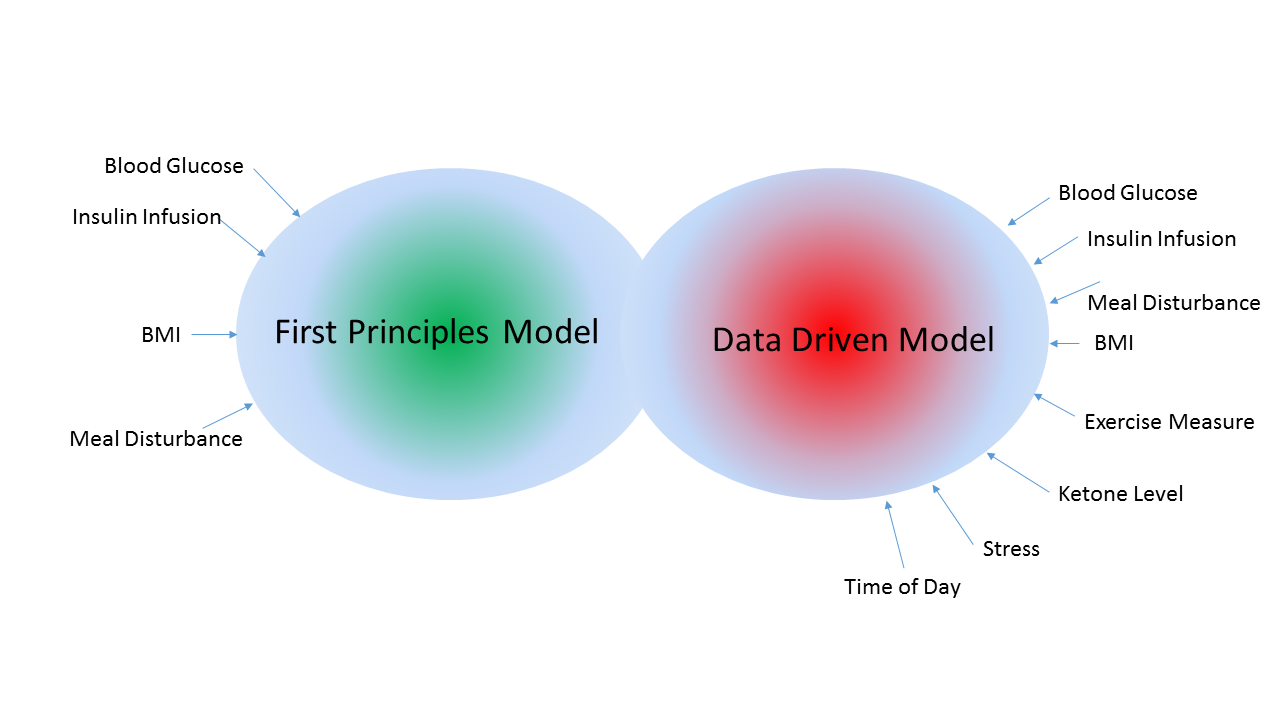
For Type 1 diabetes patients, the holy grail is an automated closed-loop therapy system, one which requires minimal user burden and can emulate a healthy person’s pancreas. With the projection of one in three children born this century developing diabetes, with 10% of them being Type 1 diabetic, the cost of the health care delivery will be a burden on the economy. Type 1 diabetes is a chronic disease and survival necessitates blood glucose regulation by external devices. With the growth of inexpensive sensors and their integration into wearable technology such as Googles smart contact lenses for glucose measurement, high resolution cameras, gyros and accelerometers in smartphone, etc., very soon the data driven dynamic system application paradigm will be the norm. The proposed work is at the intersection of data fusion, forecasting of stochastic systems and controller design which are insensitive to model parameter uncertainties. The core of the proposed work is the development of a data-driven model to identify the input-output relationship between meal input and blood glucose output as it varies over time. With the recognition that the time evolution of blood glucose for the same meal input varies across people and is a function of gender, body mass index, age, and over the short term a function of exercise, psychological stress etc. The research community over the past three decades has developed models which reasonably capture the relationship between insulin and blood glucose when subject to a meal disturbance. These models have been developed from first principles and have recently resulted in a FDA approved T1DMS (Type 1 Diabetes Metabolic Simulator) to be used in lieu of animal testing of blood glucose controllers. However, it should be noted that all the well established models for Type 1 diabetes such as the Bergman model, Hovorka model, Sorensen, Della Man model, do not account for the impact of exercise, psychological stress and other factors such as time-of-day in the determination of how the in-silico Type 1 diabetic patient will respond to meal disturbances and insulin infusion profiles. It is with the intent to develop models which can account for causal variables which are onerous or impossible to model via first principles, a data-driven paradigm will be developed building on the rich history of machine learning algorithms. Machine learning algorithms now provide startling solution to problems such as language translation, self-driving cars and object recognition. Despite their rapid growth and widespread use across a wide spectrum of applications, they have been known to be brittle, i.e., fail in scenarios for which they have not been trained. This implies for the Type 1 diabetes case, if the blood-glucose concentration in conjunction blood insulin and meal input values are outside of the range for which the data-driven model was trained, one cannot provide any guarantees about the reliability of the data-driven model. This would be of particular concern for Type 1 diabetic patients for whom the extreme scenarios of hypo- and hyperglycemia are the very situations when reliable decisions should be made by the artificial pancreas to bring the blood-glucose level to the euglycemic range. To address this shortcoming of data-driven models, this proposal will focus on development of an algorithm that fuses the data-driven model with a first-principle model with the transition defined by the confidence one has in either of the models based on the current operating domain of the artificial pancreas. The PIs will exploit the Tidepool data set to identify the data-driven model and use a statistically established validation approach to ensure the integrity of the fused model.
UBDS Contributors
Arshad Zaidi (RA), Muhanned Ibrahim (RA), Varun Chandola (co-PI)
Other Contributors
Prof. Tarun Raj Singh (PI), Dr. Lucy Mastandrea
Funding
Juvenile Diabetes Research Foundation
Project Duration
07/2019 - 12/2020